Showcasing at AAO 2024:
- ZEISS VisioGen offers a cutting-edge, AI-driven solution designed to enhance refractive patient communication and streamline clinic operations.
- ZEISS Milestone: Celebrating more than 10 million eyes treated with lenticule extraction solutions utilizing ZEISS SMILE and ZEISS SMILE pro.
- ZEISS marked its 25th year of leadership in defining optical biometry, with ZEISS IOLMaster biometers becoming the most commonly used biometers in the ophthalmic world.
- ZEISS MICOR 700 puts the future of lens extraction in the hands of surgeons as the first hand-held lens removal device with ultrasound-free operation; the FDA-cleared device is available in the U.S.
- Expanding the ZEIS
A PHP Error was encountered
Severity: Warning
Message: Undefined array key 5
Filename: views/newsdetail_view.php
Line Number: 97
Backtrace:
File: /home/judfadzm/public_html/webinar4demand.com/application/views/newsdetail_view.php
Line: 97
Function: _error_handlerFile: /home/judfadzm/public_html/webinar4demand.com/application/controllers/News.php
Line: 82
Function: viewFile: /home/judfadzm/public_html/webinar4demand.com/application/controllers/News.php
Line: 16
Function: indexFile: /home/judfadzm/public_html/webinar4demand.com/index.php
Line: 317
Function: require_once
A PHP Error was encountered
Severity: Warning
Message: Attempt to read property "image_name" on null
Filename: views/newsdetail_view.php
Line Number: 97
Backtrace:
File: /home/judfadzm/public_html/webinar4demand.com/application/views/newsdetail_view.php
Line: 97
Function: _error_handler
File: /home/judfadzm/public_html/webinar4demand.com/application/controllers/News.php
Line: 82
Function: view
File: /home/judfadzm/public_html/webinar4demand.com/application/controllers/News.php
Line: 16
Function: index
File: /home/judfadzm/public_html/webinar4demand.com/index.php
Line: 317
Function: require_once
JENA, Germany and DUBLIN, Calif., Oct. 9, 2024 /PRNewswire/ -- ZEISS Medical Technology will showcase digital enhancements and revolutionary surgical solutions at the American Academy of Ophthalmology (AAO) conference from Oct. 19-21, 2024, in Chicago. These innovations across cataract, corneal refractive, retina, and glaucoma workflows, offer new, enhanced paths to patient management and treatment, using a digitally connected environment to help advance the clinical workflow to support a higher level of personalized care.
"ZEISS continues to expand its digital leadership in ophthalmology, offering new, pioneering ophthalmic offerings and clinical tools that create an enhanced digital workflow experience for both patients and surgeons," said Euan S. Thomson, Ph.D., Head of the Digital Business Unit for ZEISS Medical Technology. "With the foundation of our Health Data Platform as part of the ZEISS Medical Ecosystem, our data-driven healthcare solutions unlock enormous value for surgeons, helping them deliver more efficient and personalized care throughout a patient's journey."
ZEISS transforms the Refractive Workflow from patient engagement to enhanced efficiency
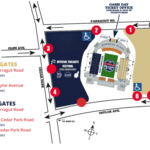
Extending the ZEISS digital portfolio, the company will introduce ZEISS VisioGen, a cutting-edge AI-driven solution designed to enhance refractive patient communication and streamline clinic operations, expanding the value to ophthalmologists and clinics by helping them grow their business through improved patient engagement. ZEISS VisioGen leverages generative AI (GenAI) for effective and efficient patient acquisition-as-a-service. The software solution provides digital communication services to offer fast, high-quality responses to patients. ZEISS VisioGen enables clinics to respond more effectively to patient inquiries while converting more patients to consultations using the latest GenAI technology combined with substantiated and verified content from ZEISS to generate personalized draft responses to patient queries for clinical staff to finalize and use.
"ZEISS VisioGen is an extremely useful tool for the busy refractive practice. It can save time and prevent grammatical errors in responding to questions from potential patients. The response time is quick and useful. Responses can also be effectively tailored to the specifics of your individual practice," said Dr. Luke Rebenitsch, MD, ClearSight, Oklahoma City (OK, USA).
Demonstrating its continued momentum in the LVC market, ZEISS recently celebrated that more than 10 million eyes have been treated with lenticule extraction solutions utilizing ZEISS SMILE and ZEISS SMILE pro, marking a significant milestone for the company and proof of the growing international adoption of safe and effective lenticule extraction solutions. In 2011, ZEISS was the first medical device manufacturer to make lenticule extraction for laser vision correction commercially available, and ZEISS SMILE and ZEISS SMILE pro continue to be leading1 solutions trusted by surgeons for the technology's reliability and effective treatment history with the VisuMax® and VISUMAX® 800 from ZEISS.
ZEISS will also showcase the recently FDA-approved VISUMAX® 800 with SMILE® pro software from ZEISS for surgically treating nearsightedness, with or without astigmatism. The updated ZEISS femtosecond laser provides U.S. refractive surgeons with faster treatment, greater flexibility, and significant workflow enhancements as compared to the previous generation VisuMax. The VISUMAX 800 creates the lenticule in less than 10 seconds by using a higher laser pulse repetition rate of 2 MHz.2 A shorter procedure time may reduce stress for both surgeons and their patients. The ZEISS femtosecond laser also provides greater flexibility for the surgeon and patient, with a smaller footprint and compatibility with a variety of patient beds, adapting to the clinical environment to provide cutting-edge technology without compromise.
ZEISS continues to reshape cataract surgery with revolutionary innovation
With a long heritage in optics, ZEISS has been shaping optical biometry for 25 years since the introduction of the first automated non-invasive optical biometer. Marking a significant milestone in cataract surgery, the ZEISS IOLMaster, was the first device to combine contactless keratometry, axial length measurement, and IOL calculation. This innovation opened the door for continuous advancements in biometry, setting a new standard for getting fewer refractive surprises. Today, the ZEISS IOLMaster is the most commonly used biometer in the ophthalmic world.
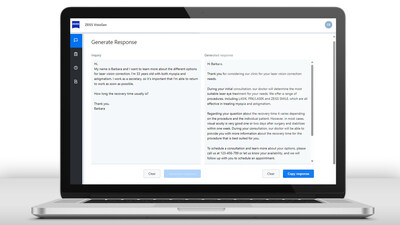
Recently FDA cleared and now broadly available in the U.S., ZEISS will demonstrate the ZEISS MICOR 700, the first hand-held lens removal device with ultrasound-free operation, reinventing lens extraction by providing a sustainable solution with a low initial investment to help surgeons expand their intraocular working space. Designed to create a gentler patient experience, the ZEISS MICOR 700 offers a revolutionary approach to lens removal, including patented crystalline lens extraction technology, a blunt and rounded tip design, and a single-use "plug & play" system with a minimal operating room (O.R.) footprint.
ZEISS will also showcase its expanded portfolio of high-end microscopes with the ZEISS ARTEVO® 850 3D heads-up ophthalmic microscope setting the pace in digital visualization with true color imaging, and increased depth of field by nearly 60 percent.3 Additionally, the ZEISS ARTEVO 850 with CALLISTO eye® features a redesigned intuitive user interface that centralizes all controls on a single touchscreen. The second latest addition to ZEISS's portfolio of optical surgical microscopes, the new ARTEVO® 750, elevates the surgical workflow by introducing advanced optical visualization technology, including new RGB LED illumination with adjustable light color temperature, as well as data overlays provided in the eyepiece with a 40 percent increase in resolution.4 The two devices are CE-marked and FDA cleared and commercially available in all major markets.
ZEISS redefines the future of retinal care, optimizing disease detection, management and treatment
ZEISS is redefining the future of retinal care, offering a holistic approach that empowers specialists to deliver excellent patient care throughout the entire patient journey. At AAO, ZEISS will showcase its comprehensive portfolio across the entire ZEISS Retina Workflow, now combining cutting-edge diagnostic solutions with advanced visualization and vitreoretinal surgical systems and next generation therapeutic lasers. The ZEISS Retina Workflow offers connected and integrated solutions that help eyecare professionals to deliver optimized patient care from early detection, through monitoring, to in-office or surgical treatments.
ZEISS will showcase cutting-edge imaging technology and 3D visualization as part of the ZEISS Retina Workflow. The CIRRUS® 6000 from ZEISS introduces an expanded Reference Database, triple that of its previous database, with greater diversity and three different disc sizes for more individualized patient care. With an acquisition speed of 100,000 A scans per second, the device provides instantaneous dense data cubes and sub-10-second-wide-field OCT Angiography capability, together with automated 9 slab standard report presentation to complement the review process. Setting the pace in digital visualization, the ZEISS ARTEVO 850 includes customizable digital color settings depending on the surgical procedure's needs and intraoperative OCT allowing for real-time monitoring of the surgical process and decision-making.
The newest addition to the ZEISS Retina Workflow, the DORC EVA NEXUS™ surgical system and instrumentation bring added value and synergy to the OR. The DORC vitreoretinal portfolio includes the EVA NEXUS™ phacovitrectomy system featuring the unique VTi pump, offering FLOW and VACUUM fluidics, EVA AVETA™ trocar cannula system with Push-Fit HI-FLOW™ infusion connection, TDC (two-dimensional cutting) vitrectomy with cut speeds of up to 20,000 CPM 5, EVA INICIO™ microinjection system, and an extensive range of posterior instruments including 27G ULTRA for no-compromise, small-gauge surgery, as well as a wide range of highly purified posterior surgical liquids and tamponades.
In addition, the next generation therapeutic laser portfolio received 510k FDA clearance for two models, the VISULAS® combi, which combines YAG and 532 modalities, and the standalone VISULAS® green (532) laser. One of the key benefits of the ZEISS VISULAS portfolio is the digital connection to the FORUM® data management solution, allowing surgeons to seamlessly integrate laser therapy into the ZEISS Retina Workflow, ensuring a high level of efficiency throughout the entire process.
ZEISS will showcase its latest offerings and new innovations at the American Academy of Ophthalmology (AAO) conference from Oct. 19-21, 2024, in Chicago at booth 4816. For more information, visit www.zeiss.com/med.
1 | Market Scope Refractive Surgery Report 2023, Global Refractive Market (Manufacturer Level) incl RLE | Refractive Market by Estimated Total Revenue, p. 273. | |
2 | Data on file, myopia with optical zone 6.5 mm. | |
3 | Compared to ZEISS ARTEVO 800. | |
4 | Compared to previous generation ZEISS LUMERA 700. Data on file. Compared to previous generation of ZEISS Integrated Data Injection Systems. Data on file. Compared to previous generation of ZEISS Integrated Data Injection Systems. | |
5 | 20,000 refers to the cuts per minute, the EVA NEXUS™ outputs 10,000 cycles per minute. | |
Not all products, services or offers are approved or offered in every market and approved labeling and instructions may vary from one country to another. For country-specific product information, see the appropriate country website. Product specifications are subject to change in design and scope of delivery as a result of ongoing technical development. The statements of the healthcare professionals reflect only their personal opinions and experiences and do not necessarily reflect the opinion of any institution that they are affiliated with. The healthcare professionals alone are responsible for the content of their experience reported and any potential resulting infringements. Carl Zeiss Meditec AG and its affiliates to not have clinical evidence supporting the opinions and statements of the health care professionals nor accept any responsibility or liability of the healthcare professionals' content. The healthcare professionals have a contractual or other financial relationship with Carl Zeiss Meditec AG and its affiliates and have received financial support. | ||
Brief Profile
Carl Zeiss Meditec AG (ISIN: DE0005313704) is one of the world's leading medical technology companies and is included in the German MDAX and TecDAX stock indices. The company supplies innovative technologies and application-oriented solutions designed to help doctors improve the quality of life of their patients. The company offers complete solutions for the diagnosis and treatment of eye diseases – including implants and consumables. In the field of microsurgery, the company provides innovative visualization solutions. With 4,224 employees worldwide, the company generated revenue totaling €1,902.8 million in fiscal year 2021/22 (ended September 30, 2022).
The company is headquartered in Jena, Germany. It has subsidiaries in Germany and abroad; more than 50 percent of its employees are based in the United States, Japan, Spain and France. The Center for Application and Research (CARIn) in Bangalore, India, and the Carl Zeiss Innovations Center for Research and Development in Shanghai, China, enhance the company's profile in these rapidly developing economies. Around 41 percent of Carl Zeiss Meditec AG shares are in free float. The remaining approximately 59 percent are held by Carl Zeiss AG, one of the world's leading companies in the optical and optoelectronic industries.
For further information visit: www.zeiss.com/med
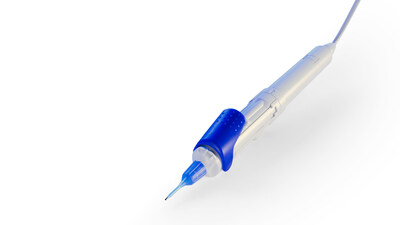
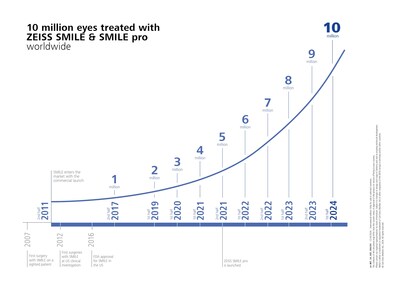

Photo - https://mma.prnewswire.com/media/2526560/ZEISS_VisioGen.jpg
Photo - https://mma.prnewswire.com/media/2526561/ZEISS_MICOR_700.jpg
Photo - https://mma.prnewswire.com/media/2526562/SMILE_10_Million_Eyes_Treated.jpg
Photo - https://mma.prnewswire.com/media/2526563/ZEISS_VISULAS_combi.jpg
Logo - https://mma.prnewswire.com/media/546786/ZEISS_v1_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/zeiss-expands-ophthalmic-offerings-to-improve-patient-care-with-new-digital-ai-tools-and-revolutionary-surgical-solutions-302271349.html
View original content:https://www.prnewswire.co.uk/news-releases/zeiss-expands-ophthalmic-offerings-to-improve-patient-care-with-new-digital-ai-tools-and-revolutionary-surgical-solutions-302271349.html